RT-PCR COVID-19 Test
RT-PCR COVID-19 Test
Test Information
Gene on Link Laboratory uses the KimForest SARS-CoV-2 Detection Kit, an FDA EUA-approved COVID-19 PCR kit, to provide accurate and efficient COVID-19 PCR testing services. The KimForest SARS-CoV-2 Detection Kit can detect new COVID-19 variants, through in-silico analysis. PCR tests are considered the gold standard for COVID-19 diagnosis due to their high sensitivity and specificity, enabling early detection even when viral load is low. Results from a PCR test can help in diagnosing active infections, guiding treatment, and preventing the spread of the virus.
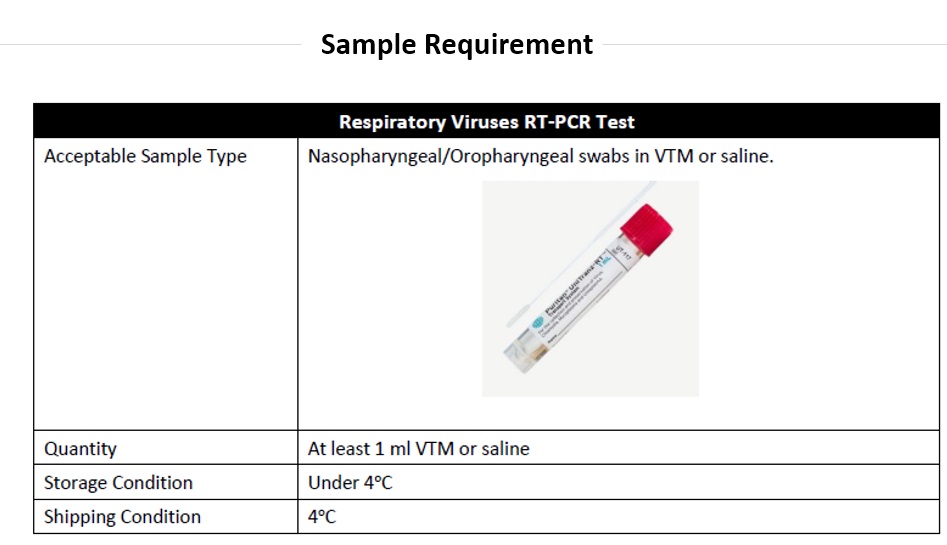
Other Test Information
- Testing Method: Reverse Transcription Polymerase Chain Reaction (RT-PCR): The test converts the viral RNA into DNA and amplifies specific genetic sequences to confirm the presence of SARS-CoV-2
- Target: RdRp gene of SARS-CoV-2 virus
- Limit of Detection (LoD): 1000 copies/mL
- Quality Control: This test detect human Ribonuclease P (RNase P) gene as an endogenous control to monitor specimen collection, nucleic acid extraction and PCR amplification.
Test Limitations and Interpretations
- A positive result likely means that the patient has or has recently had virus infection but does not always mean that the patient is still infectious.
- A negative result cannot rule out virus infection if specimen was collected over 4 days or or if the virus concentration is below the test’s LoD.
- The test results should be interpreted by qualified healthcare professionals in conjunction with clinical findings and other diagnostic information.
Gene on Link LLC. will be administered through customizable programs incorporate partnership between.
- 14738 Pipeline Ave, Ste B, Chino Hills, CA 91709, USA
- TEL:(909) 597-2789
- FAX:(909) 393-5055
Our Services
Newsletter
Do not want to miss any news, updates, notice or any offer on our products, then please subscribe to our mailing list.
Copyright by geneonlinkllc.com
