RT-PCR SARS-Cov-2/Flu/RSV Combo Test
RT-PCR SARS-Cov-2/Flu/RSV Combo Test
Test Information
The RT-PCR SARS-CoV-2/Flu/RSV Test is a precise and reliable diagnostic method used to detect the presence of SARS-CoV-2, influenza and RSV. PCR testing is highly sensitive and specific, making it an effective tool for early diagnosis, even when the viral load is low. Accurate and rapid detection of respiratory viruses enables timely treatment, helping to reduce the severity of illness and prevent its spread, especially during peak respiratory disease seasons.
Other Test Information
- Testing Method: Reverse Transcription Polymerase Chain Reaction (RT-PCR): The test converts the viral RNA into DNA and amplifies specific genetic sequences to confirm the presence of SARS-CoV-2, influenza and RSV.
- Target: SC2 genome; M&NS2 gene of Influenza virus; N gene of RSV
- Limit of Detection (LoD):SARS-CoV-2: 500 copies/mL; Influenza: 500 TCID50/mL; RSV: 125 TCID50/mL
- Quality Control:This test detect human Ribonuclease P (RNase P) gene as an endogenous control to monitor specimen collection, nucleic acid extraction and PCR amplification.
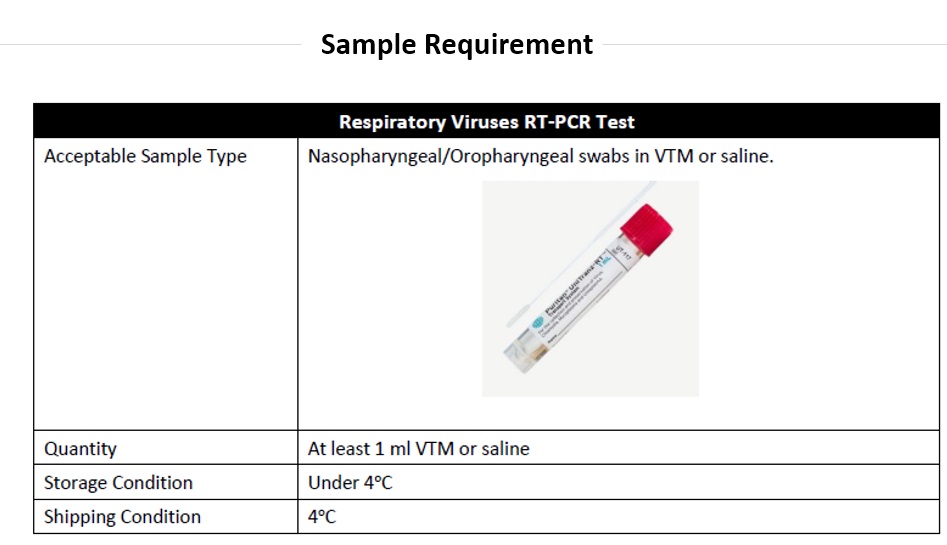
Test Limitations and Interpretations
This test cannot differentiate between influenza A and influenza B, as well as RSVA and RSVB. (If differentiation between influenza A and B is required, please order the “Influenza A/B Genotyping Test (gtFlu)” as an additional test)
A positive result likely means that the patient has or has recently had virus infection but does not always mean that the patient is still infectious.
A negative result cannot rule out virus infection if specimen was collected over 4 days or or if the virus concentration is below the test’s LoD.
The test results should be interpreted by qualified healthcare professionals in conjunction with clinical findings and other diagnostic information.
